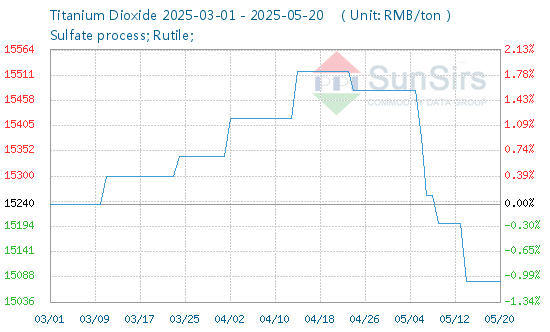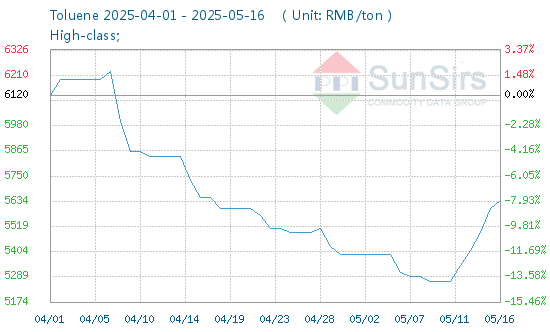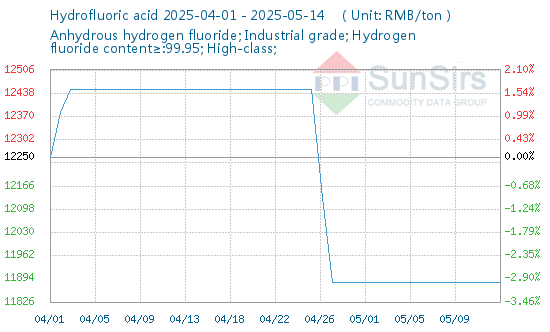

In today's chemical industry, the prominence of Monochloroacetic Acid cannot be understated. As a pivotal organic intermediate, its vast array of applications touches upon various sectors, from agrochemicals to pharmaceuticals. Delving deep into its chemistry, production, applications, and market trends, we aim to shed light on this indispensable compound and its rising significance in global industry trends.
Monochloroacetic Acid, often abbreviated as MCAA, is an essential organochlorine compound with the chemical formula C2H3ClO2. It appears as a white crystalline solid and has a sharp, pungent odor. But what's all the fuss about this particular compound? Why does it matter to industries and manufacturers? Let's dive deep and uncover the significance of Monochloroacetic Acid.
MCAA is synthesized predominantly through the chlorination of acetic acid in the presence of specific catalysts. Its production and widespread use in various industries underscore its importance, but equally noteworthy are its properties that make it a compound of choice in diverse applications.
In terms of its chemical behavior, Monochloroacetic Acid showcases remarkable reactivity, allowing it to engage in a range of reactions, from hydrolysis to nucleophilic substitutions. Its chlorinated nature means it's more reactive than its parent compound, acetic acid. This property alone grants it a special status in the world of chemical manufacturing and processing.
But, it's not just about its chemical prowess. When businesses think of monochloroacetic acid, they're also considering its economic viability, consistent quality, and the potential for scale. After all, in the competitive realm of chemical manufacturing, every edge counts. MCAA, with its unique set of properties and uses, offers just that edge to discerning manufacturers worldwide.
Monochloroacetic Acid, at its core, represents a fascinating intersection of organic and inorganic chemistry, a bridge between the realms of simple carboxylic acids and halogenated compounds. Grasping its fundamental chemistry is key to understanding its significance and diverse applications. Let's start by examining its chemical structure and formula.
The chemical formula of Monochloroacetic Acid is C2H3ClO2. Structurally, it resembles acetic acid but with a key distinction: one of the hydrogen atoms of the methyl group in acetic acid is replaced by a chlorine atom. This chlorination drastically alters its reactivity and behavior compared to its non-chlorinated counterpart.
When visualized, the molecule showcases a carboxyl group (-COOH) bonded to a carbon that's attached to both a hydrogen and a chlorine atom. The presence of this chlorine atom, a potent electronegative element, introduces unique electronic characteristics to the molecule, influencing its reactivity and interactions with other compounds.
Monochloroacetic Acid boasts a range of distinctive chemical and physical properties, courtesy of its unique structure: Chemical Properties:
Physical Properties:
Understanding these fundamental properties is pivotal for industries and researchers looking to harness the power of Monochloroacetic Acid, as they lay the foundation for its myriad of uses and applications, which we will delve into next.

The production of Monochloroacetic Acid (MCAA) is a testament to the marvels of modern chemical engineering. By harnessing various synthesis methods, industries can yield significant quantities of this compound, which plays a pivotal role in numerous applications. Let's delve deeper into the primary methods of its synthesis and the factors that influence its quality and yield.
There are several routes to produce MCAA, but some methods have been more traditionally favored due to their efficiency, scalability, and cost-effectiveness:
The production of MCAA, while straightforward, is influenced by several factors that can impact the quality and yield of the final product. Being aware of these is essential for any manufacturer looking to optimize the production process.

Monochloroacetic Acid (MCAA) is not just a mere chemical compound; it's a cornerstone for numerous industries. Its multifaceted nature allows it to be an indispensable asset in a myriad of applications. From safeguarding our crops to being integral in life-saving drugs, its contribution is vast and varied. Let's navigate through its major industrial applications.
Agriculture is the backbone of many economies, and ensuring crop protection is paramount. MCAA plays a pivotal role in the production of various agrochemicals that are vital for crop health and protection.
MCAA is used as a key precursor in the synthesis of several herbicides. These herbicides aid in controlling the growth of unwanted plants and weeds, ensuring that the crops get the necessary nutrients without undue competition. Herbicides such as MCPA and 2,4-D, used globally, owe their existence to MCAA.
Beyond just weeds, crops also face threats from various fungi. MCAA-based fungicides help in protecting crops from these fungal infestations, ensuring healthier growth and better yields. They are effective against a wide range of fungal species, making them indispensable in modern agriculture.
Healthcare, an ever-evolving domain, has found an ally in MCAA. It's extensively used in the pharmaceutical industry, both as a drug intermediate and in the formulation of therapeutic agents.
As an intermediate, MCAA is pivotal in the synthesis of various drugs. It facilitates the production of several active pharmaceutical ingredients (APIs) which are then used in formulating medications that combat a spectrum of ailments.
MCAA derivatives have direct therapeutic applications too. Certain salts and esters of MCAA exhibit medicinal properties and are used in specific therapeutic formulations, catering to niche medical needs.
Color is an integral part of our world, and MCAA ensures it remains vibrant. It plays a crucial role in the production of various dyes and pigments used in textiles, paints, and even the printing industry. The derivatives of MCAA act as intermediates, giving rise to dyes with specific hues and characteristics.
Consistency is key, whether in food, paints, or cosmetics. MCAA-derived compounds act as thickening agents, providing the right viscosity and texture to various products. These agents ensure that sauces have the right flow, paints spread evenly, and cosmetics have the perfect feel.

While Monochloroacetic Acid (MCAA) is a heavy lifter in industries, it doesn't shy away from sprinkling its magic in our daily lives too. You might be surprised to know how often you come across products influenced by MCAA without even realizing it. Let's shed light on some of these ubiquitous applications.
A radiant skin, luscious hair, or even a relaxing bubble bath, MCAA has a say in all of it. The cosmetics and personal care industry leverages the properties of MCAA and its derivatives to enhance the effectiveness of various products.
For instance, MCAA derivatives act as stabilizers, ensuring that your favorite cream retains its consistency. They also play a role in pH balancing, making sure that products are gentle on the skin. So, the next time you indulge in some self-care, remember there's a bit of science, and MCAA, pampering you.
Food is life, quite literally! And MCAA contributes to ensuring that the food we consume remains fresh and safe. It's a lesser-known fact that some salts and esters of MCAA are used as preservatives in the food industry.
These compounds ward off microbial growth, extending the shelf life of various food products. From jams to pickles, and even some beverages, MCAA's touch ensures that the food retains its taste, texture, and most importantly, its safety for consumption.
Monochloroacetic Acid (MCAA) serves numerous industries, but with its widespread use comes a responsibility to understand and manage its environmental and safety impacts. Let's delve deeper into how MCAA interacts with our environment and the precautions required when dealing with this chemical.
Being responsible means understanding the long-term effects of any chemical released into the environment. Fortunately, MCAA showcases a relatively good degree of biodegradability. When released into aquatic or terrestrial environments, it can be broken down by microbes, reducing its persistence and potential for accumulation.
However, its degradation rate can vary based on environmental conditions. It's essential for industries to monitor and manage their MCAA discharges, ensuring minimal ecological disruption.
Like many chemicals, MCAA requires careful handling and storage to ensure the safety of those working with it. Here are some general guidelines:
Exposure to MCAA can pose risks, and understanding these is crucial for ensuring safety. Some of the primary risks include:

In the vast world of organic chemistry, understanding the nuanced differences between similar compounds is paramount. Here, we'll compare Monochloroacetic Acid (MCAA) with its cousins: Acetic Acid and Dichloroacetic Acid.
MCAA and Acetic Acid share a foundational structure, but how do they differ in properties and applications?
Moving to Dichloroacetic Acid, how does it contrast with MCAA?
The dynamics of the global market for Monochloroacetic Acid (MCAA) are influenced by a combination of technological advancements, evolving industry needs, and shifts in regional production and consumption. Let's take a closer look.
Regional dynamics play a pivotal role in the MCAA market, with some regions emerging as significant producers and consumers.
As with any industry, the MCAA market faces a horizon shimmering with opportunities and challenges.
In the evolving tapestry of the global chemical industry, Monochloroacetic Acid and its diverse applications stand out as a testament to its unparalleled importance. For businesses, researchers, and stakeholders, understanding its nuances offers a competitive edge. As China's leading manufacturer of Monochloroacetic Acid and Organic Intermediates, we invite you to explore more and collaborate with us at HighMountainChem. – where innovation meets excellence.